The EFSA’s role in Food and Feed Enzymes and Additives
Enzymes are proteins that act as catalysts to speed up chemical reactions. They are widely used in food/feed processing.
Additives are substances added to food or feed to improve animal health, productivity, or feed/food quality.
Safety of the substance must be assessed before its use in products. Several aspects of the safety are challenged. If the substance is produced or consists of a microorganism, it is asked to prove its microbial safety.
Other categories are considered, as:
environmental safety

health safety (Human or animals)

consumer safety (when the animal fed is then consumed)

workers safety (ensure that workers in direct contact with the substance are safe)

dietary exposure (quantity ingested by the consumer)…
Efficacy of the substance is also assessed for the Feed additives, to assess whether they actually deliver the claimed benefits.
The EFSA’s role in Novel Food
Novel Food is defined as food that was not significantly consumed in European Union prior to 15 May 1997.
As with enzymes and additives, EFSA assesses Novel Foods prior to their potential marketing authorisation. Depending on their category, a more in-depth assessment is required to certify their safety. In fact, their nature can lead to challenges to current regulations, such as foods derived from cell culture or foods resulting from industrial fermentation.
Some EFSA’s figures
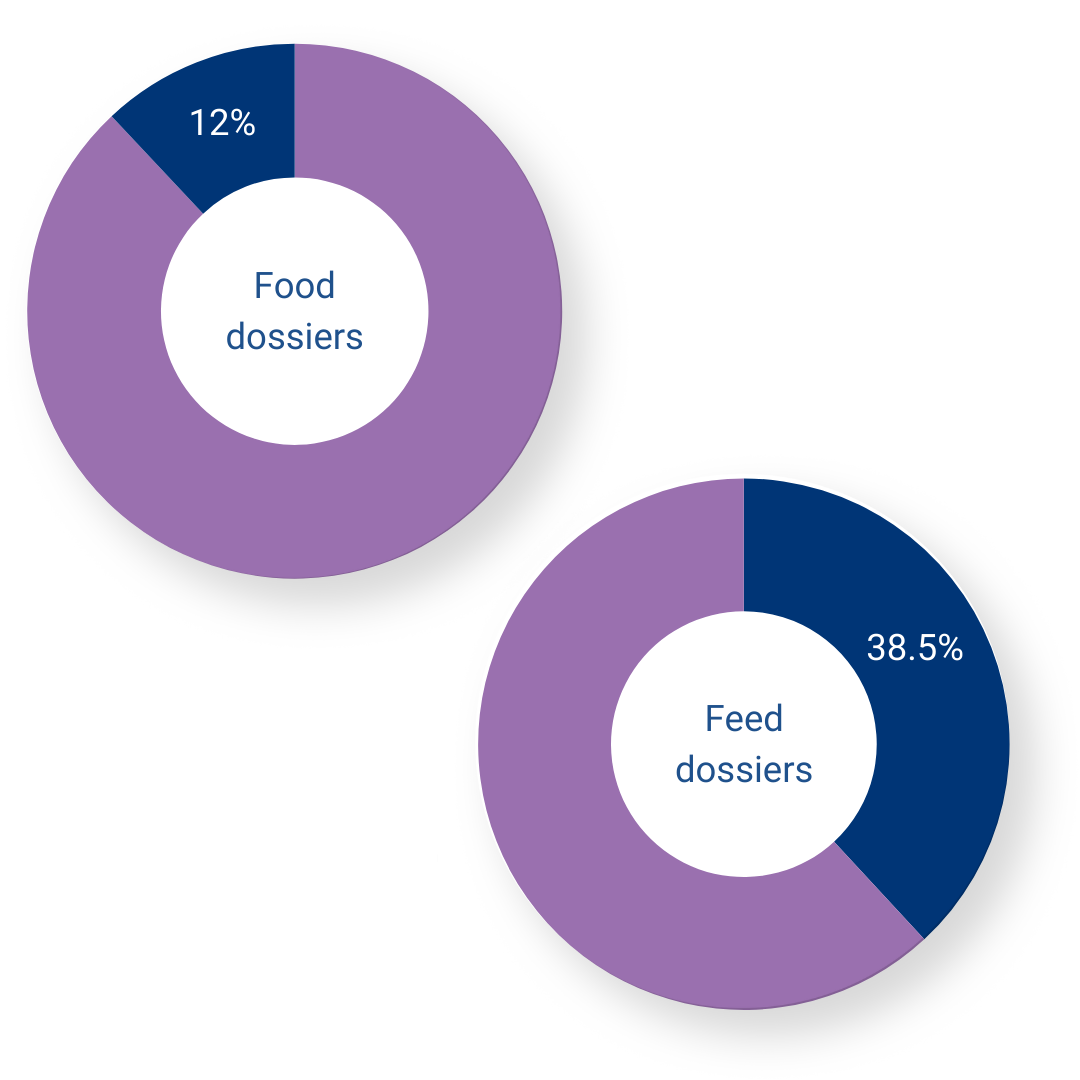
In one year, the EFSA reviewed 83 Food dossiers for products involving microorganisms, coming from a total of 18 industrials. They also reviewed 78 Feed dossiers involving microorganisms, handed by 41 industrials.
They rejected 12% of the Food dossiers, and 38.5% of the Feed dossiers, mostly for issues with data and experimentation designing issues than for health threat.
About us
GenoScreen developed its expertise in regulatory affairs to help its clients and prospects comply with the expectations of the regulatory bodies. We run dossiers compliant testing including genomic characterization of microbial strains. We accompany our clients eager to fill in safety dossiers when it comes to microbiological analyses by providing them EFSA compliant microbiological reports. By doing so, we help them improving their success odds, saving time and relieving regulatory burden.
The GenoScreen experience in a nutshell...

From setting up your project to delivering the results

Methods and solutions tailored to your projects

Micro-organism studies for over 20 years

Support beyond results delivery

‘materials and methods’ explained